- Home > Glass Vials >
- 30ml (30R) Sterile Empty Sealed Vials
Print
-
30ml (30R) Sterile Empty Sealed Vials
-
20ml (20R) Sterile Empty Sealed Vials
-
50ml (50R) Sterile Empty Sealed Vials
-
10ml (10R) Sterile Empty Sealed Vials
-
6ml (6R) Sterile Empty Sealed Vials
-
100ml (100R) Sterile Empty Sealed Vials
-
Specimen Collecting Vials
-
Universal Sampling Vials
-
Injection Vials - Polypropylene
-
Infusion Vials - Polypropylene
-
Dropper Vials
-
Transportation Vials
-
Scintillation Vials
-
Headspace Vials Screw Top
-
Sterile Empty Sealed Vials (2R to 100R)
-
Injection Vials - Tubular - Amber
-
Injection Vials - Tubular - Clear
-
Injection Vials - Molded - Clear - Type III
-
Injection Vials - Molded - Amber - Type III
-
Injection Vials - Molded - Amber - Type 1
-
Injection Vials - Molded - Clear - Type 1
-
Infusion Vials - Molded - Clear - Type 1
-
Headspace Vials Crimp Top
-
Shell Vials
-
Vanity Vials
-
EPA-VOA Vials
-
Sample Vials, Wide Mouth
-
Diagnostic Screw Neck Vials
-
Dram Vials - Clear
-
4ml/13mm Screw Top HPLC Vials
-
2ml/9mm Crimp Top HPLC Vials
-
2ml/9mm Screw Top HPLC Vials (Pre-Slit)
-
2ml/9mm Screw Top HPLC Vials
-
2ml/8mm Screw Top HPLC Vials
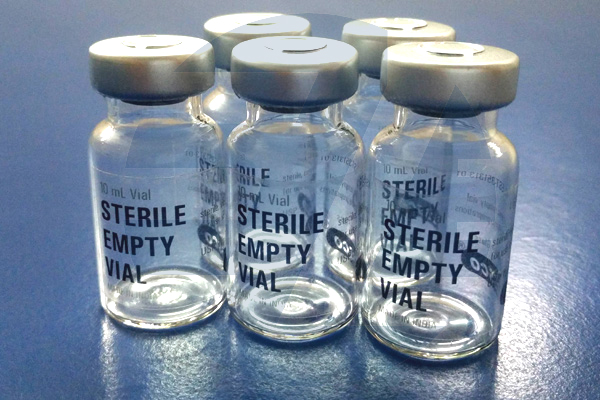
- Essco Brand 30ml (30R) Empty Sealed Sterile Vials. (Product Code: ES.11619.00030)
- Each phase of our production process is conducted in cGMP compliant facility equipped with the latest generation equipment within certified clean rooms [ ISO 8 (class 100,000), ISO 7 (class 10,000), ISO 5 (class 100)] using validated processes.
- Vials are washed with lab grade distilled water and then by Isopropyl Alcohol (IPA) before Dry Heat Sterilization.
- After Dry Heat Sterilization, each vial is sealed with steam sterilized (autoclaved) rubber stopper and steam sterilized (autoclaved) aluminum seal and then further packed in tamper proof Ethylene Oxide (ETO) sterile plastic bags to maintain sterility during storage and transit.
- These tubular USP/EP TYPE I injection vials are formed as per international DIN/ISO 8362-1 standards and are available in 6R, 10R, 20R, 30R, 50R and 100R sizes. Amber glass vials also available for bulk orders.
- Sterility Test Report from NABL Accredited Lab available at extra cost.
- Buy from Amazon
30ml (30R) Sterile Empty Sealed Vials

- Essco Brand 30ml (30R) Empty Sealed Sterile Vials. (Product Code: ES.11619.00030)
- Each phase of our production process is conducted in cGMP compliant facility equipped with the latest generation equipment within certified clean rooms [ ISO 8 (class 100,000), ISO 7 (class 10,000), ISO 5 (class 100)] using validated processes.
- Vials are washed with lab grade distilled water and then by Isopropyl Alcohol (IPA) before Dry Heat Sterilization.
- After Dry Heat Sterilization, each vial is sealed with steam sterilized (autoclaved) rubber stopper and steam sterilized (autoclaved) aluminum seal and then further packed in tamper proof Ethylene Oxide (ETO) sterile plastic bags to maintain sterility during storage and transit.
- These tubular USP/EP TYPE I injection vials are formed as per international DIN/ISO 8362-1 standards and are available in 6R, 10R, 20R, 30R, 50R and 100R sizes. Amber glass vials also available for bulk orders.
- Sterility Test Report from NABL Accredited Lab available at extra cost.
- Buy from Amazon
Copyright 2025 esscoglass ,All Rights Reserved | Designed & Developed by DOT Technologies
Verify Your Email
Resend Code
Please enter your email address
Please enter Varification Code
Get Verification Code
Verify







